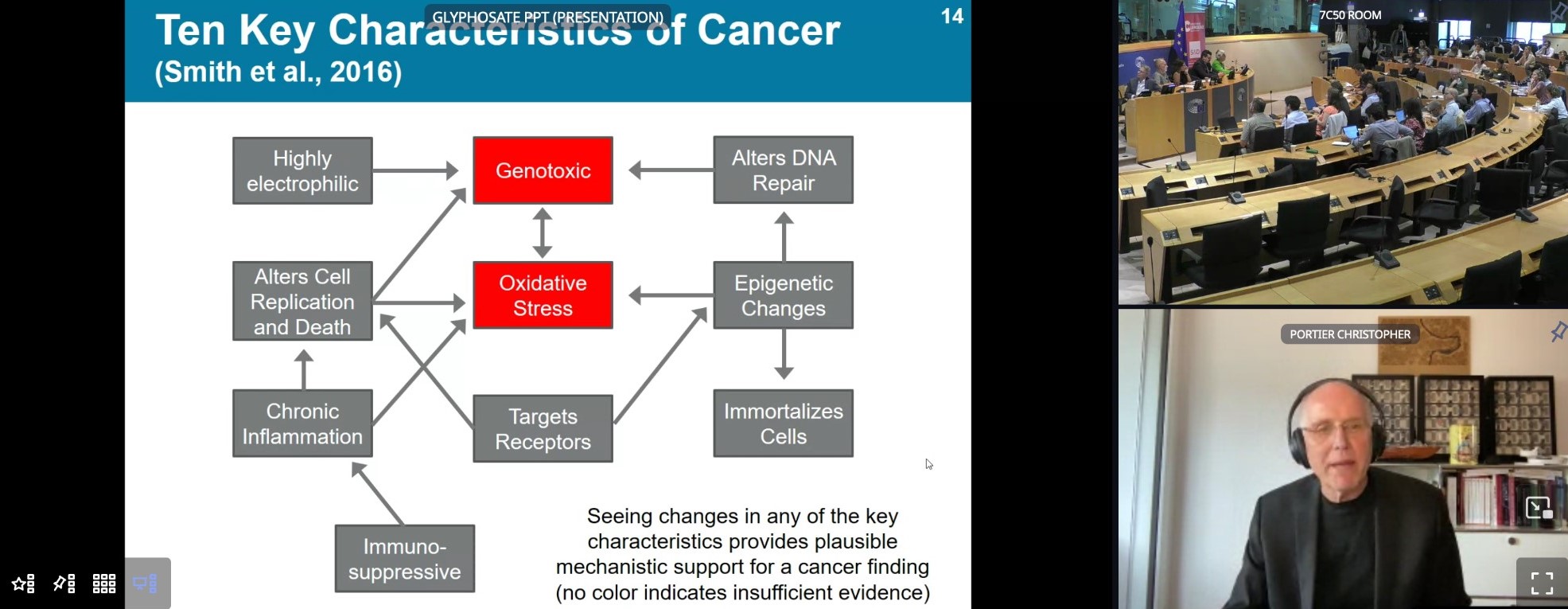
Genotoxicity, carcinogenicity, damage to the gut microbiome and endocrine disruption by glyphosate and glyphosate-based products have been given little importance in the safety assessment by ECHA and EFSA. The same is the case for harmful effects on soil, water and biodiversity. This was highlighted during a meeting with several scientific experts in the EU Parliament on September 18th 2023. The gaps are partly due to important flaws and partly to the outdated and biased, industry-friendly approach in the guidelines for these assessments. A review of the most recent independent science gives a completely different picture on the harm done by glyphosate and its formulations. Professor Michael Antoniou from King's College London sighed: “When will regulators stop living in the dark ages and come into the 21st century?”
The event was organised by PAN Europe in cooperation with EU Parliament members Christophe Clergeau (S&D) and Jutta Paulus (The Greens/EFA). From ECHA Mike Rasenberg, Director, Hazard Assessment and Paul Ryan, Head of Unit, Hazard I took part. EFSA was represented by Manuela Tiramani, Head of Pesticides Peer Review Unit. Klaus Berend, Head of Unit, Pesticides and Biocides from DG SANTE spoke on behalf of the European Commission.
Medical doctor Daniele Mandrioli gave an insight into the procedures and first findings of the Global Glyphosate Study. This independent study is the most comprehensive one ever done on glyphosate and its formulation MON52276 (Roundup Ultra) generally used in Europe. While the full results on carcinogenicity will be presented in late October, Dr Mandrioli already explained that they observed serious adverse effects such as indications of endocrine disruption, reproductive effects during development, microbiome alterations and genotoxicity. These effects were observed with glyphosate alone and were more prominent with the formulation.
Professor Michael Antoniou showed that the methods used by the EU institutions to conclude on glyphosate’s safety are outdated and insufficient. Regulators should also focus on the impacts of the whole product and not only of glyphosate alone, as the added ingredients (like co-formulants) may increase the toxicity of the product. His studies have shown that glyphosate and its representative formulation MON 52276 cause changes in the gut microbiome at very low doses that are considered safe by EU Regulators. Exposure also caused oxidative stress in blood and gut, an indication for damage to cells, organs and even DNA that can lead to cancer. The discovery that glyphosate damages DNA alone should, under EU pesticide law, result in a ban on the chemical. The studies of his team were not included in the evaluation of all the evidence by the authorities.
Professor Christopher Portier explained that both epidemiology and animal cancer studies found positive association between glyphosate use and cancer. Dr. Peter Clausing, one of the authors of a recent study by toxicologists and cancer experts revealed that ECHA dismissed important carcinogenicity findings and neglected evidence that glyphosate induces oxidative stress, a recognised mechanism that can lead to cancer. EFSA, in its conclusions, wrongfully relied on ECHA's classification of glyphosate as 'non-carcinogenic'. This is a critical failure since accepting this scientific evidence would inevitably lead to the conclusion that the glyphosate authorisation cannot be prolonged under EU law.
Ecologist Dr. Johann Zaller heavily criticised the lack of conclusions on biodiversity by EFSA, saying “Such a conclusion is a disrespect of biodiversity and ecological science.”
Xavier Reboud from the French INRAE institute explained why many farmers still prefer to use glyphosate. He showed that there are viable alternatives for quite some uses while farmers need support to change in more complex situations.
Dr. Angeliki Lysimachou from PAN Europe concluded that the scientific literature indicates serious adverse effects following exposure to glyphosate-based herbicides. Leaving the carcinogenicity potential of glyphosate aside, even EFSA acknowledged that certain ingredients of glyphosate have shown genotoxic or carcinogenic potential or have data gaps, and glyphosate based products may cause neurotoxicity, disruption of the microbiome or have an impact on biodiversity. In fact a long term toxicity study on the formulation has not been performed by the applicants. Based just on this evidence and the provisions of EU law and case law, glyphosate’s approval should not be renewed as a high level of protection from human health and the environment has not been demonstrated.
The representatives of ECHA and EFSA stated that many scientists were involved and hundreds of studies were assessed, while they followed the regular proceedings as required by the guidelines and have come to a well-balanced conclusion. Mr Klaus Berend stated on behalf of the European Commission that no major concerns were found and the renewal proposal will be published soon, which Member States will be invited to vote on in October.
Summaries and link to the presentations below
Evaluating the carcinogenicity of glyphosate
Christopher J. Portier, Former Director of the US National Center for Environmental Health
Professor Christopher Portier is former Director US National Center for Environmental Health. He exposed that the majority of the published epidemiology studies found an association between glyphosate and cancer, furthermore all published Meta-Analyses found this association.
He highlighted that ECHA’s opinion highly relied on a study by Andreotti et al. (2018). This study made a misclassification in exposure, which “reduces the risk estimate, potentially to below zero”. Portier stated that both epidemiology and animal cancer studies found positive association between glyphosate formulation use and cancer. Both alone would justify glyphosate to be classified under CLP to 1B “Limited” evidence of carcinogenicity from epidemiology studies and “sufficient” evidence from animal studies, along with evidence indicating the underlying biological mechanism.
So his answer to the conference question ‘Is glyphosate safe for health and the environment?’ was clear: “NO, It can cause cancer in humans.”
The majority of the published epidemiology studies found an association between glyphosate and cancer.
Summary:
- All published Meta-Analyses found association between glyphosate and cancer.
- A large number of Exposure-Time-Response studies found association between glyphosate and cancer.
- ECHA relies a lot on Andreotti et al. (2018) - Which is misclassification in exposure. About 1/3 of the participants did not respond to the questionnaire and their exposures were “imputed” using a failed statistical model. This misclassification reduces the risk estimate, potentially to below zero. This study should be given little or no weight in the evaluation.
- Epidemiology: Positive association exists between glyphosate formulation use and NHL - with credible causal inference. ECHA CLP Designation for the epidemiology alone would be “Limited Evidence of Carcinogenicity” - 1B
- Animal Cancer Studies: Positive association exists between glyphosate formulation use and an increase in carcinogenicity in two or more independent cancer studies in animals with credible causal inference. ECHA CLP Designation for the animal cancer data alone would be “Sufficient Evidence of Carcinogenicity” - 1B
- Glyphosate has at least two key characteristics that provide support on the underlying mechanism that may lead to cancer
Presentation: Evaluating the Carcinogenicity of Glyphosate
Glyphosate and ECHA's "weight of evidence"
Dr Peter Clausing, senior toxicologist, glyphosate expert for PAN Germany

Peter Clausing is a toxicologist with a career in regulatory toxicology. Currently an expert at PAN Germany, he is the co-author of a recent study on oxidative stress and glyphosate. Clausing highlighted that ECHA’s Opinion, according to which glyphosate does not meet the criteria to be classified as a potential carcinogenic, is unscientific. He provided compelling evidence that ECHA dismissed important elements of the Weight of Evidence, by making untrue statements to support its position. In essence, he showed that the agency has wrongly dismissed tumours in carcinogenicity studies based on a flawed justification. In violation of applicable OECD guidance for the analysis of tumour data, ECHA has favoured a method of statistical evaluation which showed non-significant results for tumour data, and used a top limit dose that does not exist for carcinogenicity. The agency has wrongfully claimed that kidney tumours “were not likely to be treatment-related, because … there was no plausible mechanism”, where in fact there is evidence that glyphosate causes oxidative stress, a mechanism that can lead to DNA damage.
While ECHA did not propose a classification for glyphosate’s carcinogenicity, for Clausing, there is sufficient evidence to classify glyphosate as a presumed human carcinogen (category 1b), or at the very least as a suspected human carcinogen (category 2).
- For the 5 independent carcinogenicity studies in mice for glyphosate, there was an increase in tumor incidences in the majority of studies. Nevertheless, ECHA concluded that this is “not likely to be treatment related”.
- ECHA has wrongly dismissed 2 of the studies and declared them not relevant for the assessment, based on a limit dose that has been defined for chronic toxicity studies, NOT for carcinogenicity studies. The two studies should have been considered relevant
- ECHA only used the non-significant results of pairwise comparisons ( a method of statistical evaluation), in violation of applicable OECD guidance for the analysis of tumour data. OECD explicitly recommends trend tests (the other method of statistical evaluation) and states that “Significance in either kind of test is sufficient” to recognize the result
- Dr Clausing showed that ‘Historical Control data’ support the study findings, thus invalidating ECHA’s claim that the kidney tumours “were not likely to be treatment related, because … the findings were within the historical control ranges.”
- ECHA claims that the kidney tumours “were not likely to be treatment related, because … there was no plausible mechanism”. Dr Clausing showed that oxidative stress is a plausible mechanism for the kidney tumours.
There is sufficient evidence for a category 1B classification of glyphosate. Nevertheless a category 2 classification should be more than justified:
- According to ECHA, Category 2 is adequate, if “evidence of carcinogenicity is restricted to a single experiment”. In our case we have 5 studies with increased tumour rates, and in our example 3 of 5 studies with kidney tumours – statistically significant, supported by HCD and supported by a plausible mechanism;
- According to ECHA, Category 2 is adequate, if there are “unresolved questions regarding … the design, conduct or interpretation of the studies”. Here, we have been talking about 5 studies declared “valid” by ECHA.
- And finally, Category 2 is adequate, if a substance causes increased rates only of benign tumours. Here we have malignant tumours in all 5 studies, including renal carcinoma.
ECHA’s decision for no classification of glyphosate is unscientific, and based on untrue statements to support it.
Presentation: Glyphosate and ECHA's "weight of evidence" by Dr. Peter Clausing.
The Global Glyphosate Study
Daniele Mandrioli M.D, Ramazzini Institute
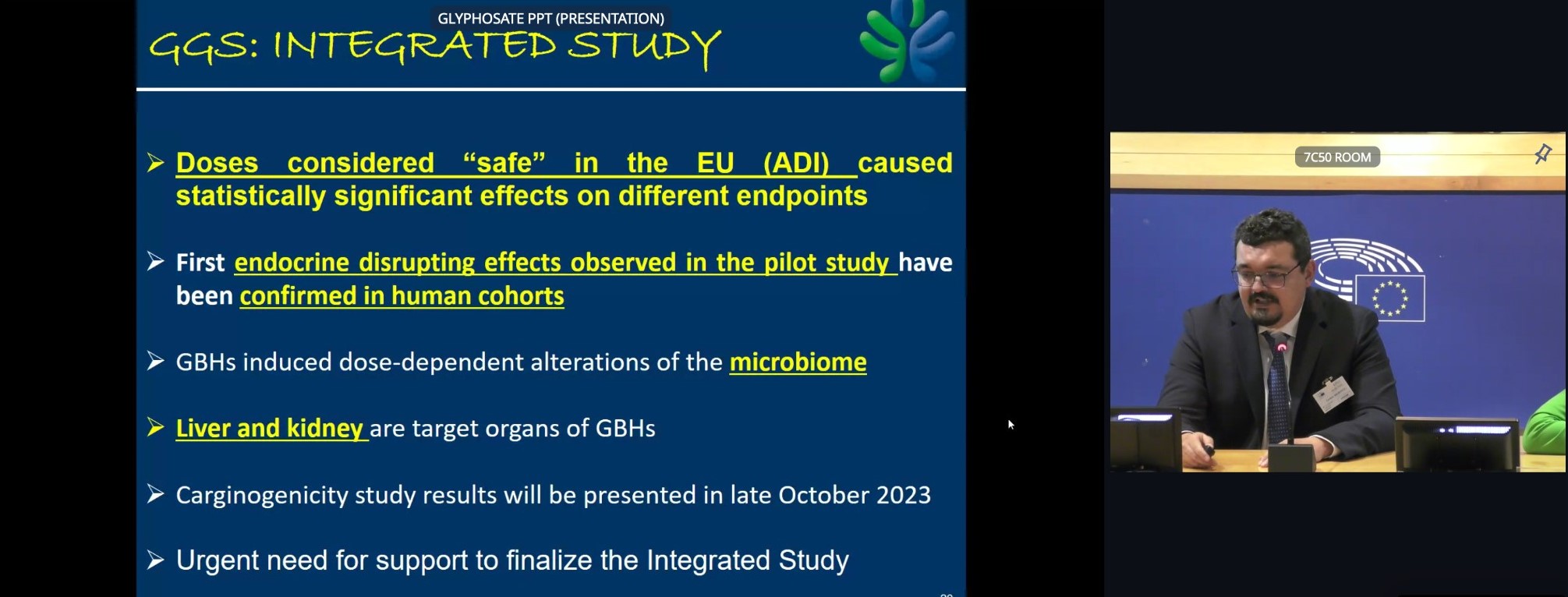
Daniele Mandrioli, Medical Doctor and Director of the Cesare Maltoni Cancer Research Center at the Ramazzini Institute presented the The Global Glyphosate Study. This is the most comprehensive study on glyphosate and its products by a range of renowned university institutes in different countries. They found statistically significant effects on different endpoints at doses considered safe in the EU evaluation. Endocrine disrupting effects were observed in the pilot study and confirmed in a human cohort study. They also found an increase in micronuclei (biomarker of genotoxicity). Micronuclei increase is frequently associated with substances that are capable of inducing lymphoma or leukaemia. Dose dependent alterations of microbiome, for both glyphosate and formulation were observed, with the formulation generally having more detrimental effects. The results of The Global Glyphosate Study will be presented at the end of October.
The study is based on a pilot phase (one dose, 2 test substances, limited endpoints), and an integrated study (3 doses, 3 test substances and multiple endpoints)
Findings of pilot study :
- Different concerns on microbiome and endocrine effects
- Found alterations of microbiome when exposure started from pregnancy
- Not only microbiome, alteration of the metabolome, connected to microbiome alteration
- Increase micronuclei (biomarker of genotoxicity), at doses allegedly safe. Micronuclei increase is frequently associated with substances that are capable of inducing lymphoma or leukemia.
- Increase of anogenital distance, along with increase of testosterone in females, along with a delay in the first testeros in females.
Findings confirmed in humans, in a very large cort, multicentric study. Anogenital distance increase in newborns exposed during pregnancy. Level of maternal urinary exposure, higher levels associated with anogenital distance.
Observed alteration of the length of gestation in mothers exposed during their pregnancy to glyphosate.
Integrated phase of the study. Acceptable daily intake (0.05mg/kg body weight) study EU formulation and US formulation (with POEA). Up to 50 mg/kg, a dose which should give no adverse effects. 9 treated groups, one control. Many outcomes.
- Observed alterations of microbiome, at doses considered safe, with a dose response
- Observed alteration of kidney and liver (subchronic toxicity study - prenatal exposure to 13 week of age)
To summarise, we observe that doses considered safe in the EU cause statistically significant effects on different endpoints : endocrine disrupting effects, observed in pilot study and confirmed in human cohorts. Dose dependent alterations of microbiome, for both glyphosate and formulation, with the formulation generally having more detrimental effects. Carcinogenicity results will be made available in October.
Presentation: The Global Glyphosate Study
Multiple organ system toxicity of glyphosate and Roundup Bioflo (MON 52276) at regulatory relevant doses
Professor Michael Antoniou, Head of the Gene Expression and Therapy Group at King's College London

Professor Michael Antoniou, the Head of the Gene Expression and Therapy Group at King's College in London highlighted the results of two major rat studies on glyphosate and the glyphosate formulation (MON 52276) indicating serious damage to health. The studies have not been included in the ECHA and EFSA opinion.
The findings highlight microbiome alterations following exposure to very low, in theory “safe” doses of glyphosate or its representative formulation. Oxidative stress response has been found in both the gut and blood, with both glyphosate and Roundup MON 52276 which was more disruptive than glyphosate. Oxidative stress damages: cells, organs and DNA that can lead to cancer. Michael Antoniou reminded that farmers never use active ingredients alone. He stressed the importance of testing glyphosate formulations that contain the active substance together with co-formulants that can increase the toxicity of the product.
To regulators, the scientist asked why they fail to require meaningful toxicological data, including long-term testing, on co-formulants but also why his study was not included in ECHA’s opinion on glyphosate toxicity. He stated that damages to DNA should, under EU pesticide law, result in a ban on the chemical.
Summary:
- Their study found: All doses of glyphosate and Roundup MON 52276 tested caused some adverse effects in rats; current EU glyphosate / Roundup NOAEL and ADI are incorrect.
- Glyphosate and MON 52276 disturb gut microbiome at doses regulators say are safe - by the same mechanism by which the chemical acts as a weedkiller – inhibition of the shikimate biochemical pathway. Humans/animals don’t have the shikimate pathway, enabling industry and regulators to state that glyphosate is nontoxic to humans. But fungi and some strains of gut bacteria do have this pathway.
- They altered the microbiome at all doses tested, even at low (“safe”) doses (NOAEL, ADI).
- They decreased bacterial diversity and increased fungal diversity
- Oxidative stress response in both the gut and blood, with Roundup MON 52276 more disruptive than glyphosate. Oxidative stress damages: cells, organs, DNA that can lead to cancer.
- Epidemiological studies show oxidative stress and DNA damage response following occupational exposure to GBH
- Glyphosate and Roundup MON 52276 damaged the rats’ livers.
- Standard tests: dose-dependent adverse effects from Roundup MON 52276 - and statistically significant increase in fatty liver disease and liver cell death
- Modern non-standard tests (not considered by regulators): MON 52276 altered the expression of 96 genes in the liver linked to oxidative stress and DNA damage, as well as disruption of circadian rhythms ("body clocks”).
- Direct DNA damage in the liver increased with glyphosate exposure.
- Discovery that glyphosate alone damages DNA should, under EU pesticide law, result in a ban on the chemical.
- Epigenetic changes suggest cancer-causing ability
- Glyphosate damaged DNA in living animals but not in the cell system. This shows that in vitro tests are not enough!
- Farmers never use active ingredients alone. Stronger adverse effects were seen from Roundup MON 52276 than glyphosate; why do regulators fail to require meaningful toxicological data, including long-term testing, on co-formulants?
- Collectively all measures point to glyphosate, and more so to Roundup, as potent risk factors for fatty liver disease and carcinogenicity
- Adverse effects from glyphosate/Roundup exposure are slow to become evident and may give a false sense of safety. In their study adverse effects were revealed even at 90 days because as more sensitive techniques than the standard toxicity tests were used to analyse the composition of blood and contents of the gut. Tests required by regulators will miss important toxic effects.
- “When will regulators stop living in the dark ages and come into the 21st century and demand molecular profiling methods to be used in risk assessment?
- Why were our studies presented here not included in the ECHA opinion on glyphosate toxicity?”
Presentation: Multiple organ system toxicity of glyphosate and Roundup Bioflo (MON 52276) at regulatory relevant doses
Ecotoxicity of glyphosate
Professor Johann G. Zaller, Institute of Zoology, University of Natural Resources and Life Sciences Vienna - author of the ecotoxicological review of glyphosate


Ecotoxicity of glyphosate - Johann Zaller, from the Institute of Zoology, University of Natural Resources and Life Sciences Vienna highlighted the unacceptable risks of glyphosate to the already alarmingly damaged biodiversity. Zaller explained that glyphosate and its formulations have unintended side-effects on many terrestrial organisms including mammals. The mechanisms are known, like oxidative stress with effects on biochemistry and DNA damage.
He stated that official risk assessment is not assessing what is happening in the field in reality, as most studies focus only on short-term effects of a single glyphosate application, or its products to effect to a single species. In reality 2 to 3 glyphosate applications happen in one season, and there are interactions with other agrochemicals, and also there are other ecological interactions.
Zaller reminded the conference audience that glyphosate is already everywhere in the environment, the most frequently found pesticide as a pollutant in our soils. He quoted PANs surface water study and showed that glyphosate was even found in the centre of Vienna and in the air of Austrian National Parks. Zaller pointed out how glyphosate impairs ecological interactions between species and even the nutrient content of crops.
He quoted EFSA’s assessment that glyphosate poses “high long-term risks to mammals”, and reminded us that “we are mammals”. He heavily criticised the lack of conclusions on biodiversity by EFSA, saying “Such a conclusion is a disrespect of biodiversity and ecological science.”
Summary:
- There are several threats to biodiversity, and in most factors glyphosate is also involved (Changes in land and sea use, habitat loss, degradation; Invasive species and disease; environmental pollution)
- Glyphosate & formulations have unintended side-effects on many terrestrial organisms including mammals. Through different mechanisms:
- oxidative stress with effects on biochemistry and DNA damage.
- disruptions of various physiological, behavioral and ecological processes.
- Official risk assessment is not assessing what is happening in the field: most studies only short-term effects of a single glyphosate application, or GBH to a single species. Reality: 2-3 glyphosate applications per season, interactions with other agrochemicals, and ecological interactions. Limited assessment on very few surrogate species
- GBH are more toxic than glyphosate & arsenic, chromium, cobalt, lead and nickel.
- Glyphosate is everywhere
- contaminates aquatic ecosystems - Glyphosate may pose a moderate to high risk in 95% of countries investigated. PAN study - Glyphosate and/or AMPA were detected in 74% of the samples, in 11 out of the 12 countries
- 80% of soils with pesticide contamination. Most frequently found: Glyphosat (+ AMPA), DDT
- glyphosate in air in two National Parks and incenter of Vienna
- Glyphosate affects plant defense and health
- Effects of the metabolism of fungi and bacteria (shikimate pathway)
- Effects on interaction between plants and microorganisms: chemical signaling, pollinators, herbivores, .
- Glyphosate impairs the nutrient content of crops
- Glyphosate kills all plants and impairs ecological interactions between species.
- Glyphosate “high long term risks to mammals” - we are mammals
- EFSA’s (no) Conclusion on biodiversity: “SUCH A CONCLUSION IS A DISRESPECT OF BIODIVERSITY AND ECOLOGICAL SCIENCE”
Presentation: Ecotoxicity of glyphosate
Alternatives to glyphosate
Xavier Reboud, INRAE, France


Dr. Xavier Reboud is a researcher in Agroecology at INRAE, a French public research institute. He studies agricultural practices that reduce dependence on pesticides and coordinated a study on the case of glyphosate. Reboud explained why farmers of annual field crops and orchards and vineyards see advantages of glyphosate. They can often do without glyphosate in the long term, but many farmers find it important to keep glyphosate as a security in case of difficult years or the emergence of more difficult-to-control weeds. The economic impact of doing without glyphosate for them often appears moderate.
For some uses of glyphosate there is a non-chemical alternative in common use and with no major economic impact. In other uses, farmers need support to solve situations where going without glyphosate is difficult. This is in particular in cases where it is not technically possible or desirable to take preventive measures or use mechanical weeding.
Summary :
- At European level, depending on the crop, yearly, between one-third and one-half of plots are glyphosate-free. However, this does not mean that it is possible to do without it every year as it can mean that all plots receive glyphosate every two yeasr or every three years. This is often the case, but at the same time farmers emphasize the importance of maintaining this security in case their situation deteriorates due to a combination of difficult years or the emergence of weeds such as thistles, which are more difficult to control.
- ANSES concludes (2020) that there are uses for glyphosate for which there is a non-chemical alternative that is in current use and has no major economic impact.
- An analysis of the different situations in which farmers work reveals a range from zero to high dependence on glyphosate, depending on the situation. Stopping the use of glyphosate ranges from simple and easy to very complex
- Difficulties result from the changes induced by Glyphosate and which are difficult to reverse: e.g. increase in farm size, changes in land use and rotations,scarcity of labor shortages, desire to conserve land, etc.
- The economic impact often appears moderate, partly transitory (e.g. time for perennial crops to adjust their root system, compensated through better anticipation by (e.g. through varietal choice, the vigor of the rootstock, etc)
- The first alternative is mechanical weeding (soil work), but there are other possible levers. Others still need to be explored/improved > extended responsibility of actors upstream and downstream of agriculture
- Depending on the future of glyphosate in Europe, there will be actions to consider: management of impasses in the event of withdrawal, trade-offs on the most important uses only ?
Presentation: Alternatives to glyphosate
A civil society perspective
Dr. Angeliki Lysimachou, PAN Europe
Angeliki Lysimachou is an Environmental scientist and toxicologist, Head of Science and Policy at Pesticide Action Network (PAN) Europe. She has been closely following the EU assessment of glyphosate since 2014.
The EU Regulation on plant protection products aims to ensure a high level of protection for human, animals and the environment:
- Cut-off criteria as set for hazardous substances (e.g. mutagens, carcinogens, toxic to reproduction, endocrine disruptors, POPs, PTBs)
- Particular attention should be given to the protection of most vulnerable groups like children, as well as impacts to ecosystems and biodiversity
- The industry must demonstrate that active substance, the product, all its ingredients and residues in food and the environment cause no harm
- The assessment has to be independent, objective & transparent, with no ties to the pesticide industry, including the most up to date science, such as the one from peer reviewed scientific literature
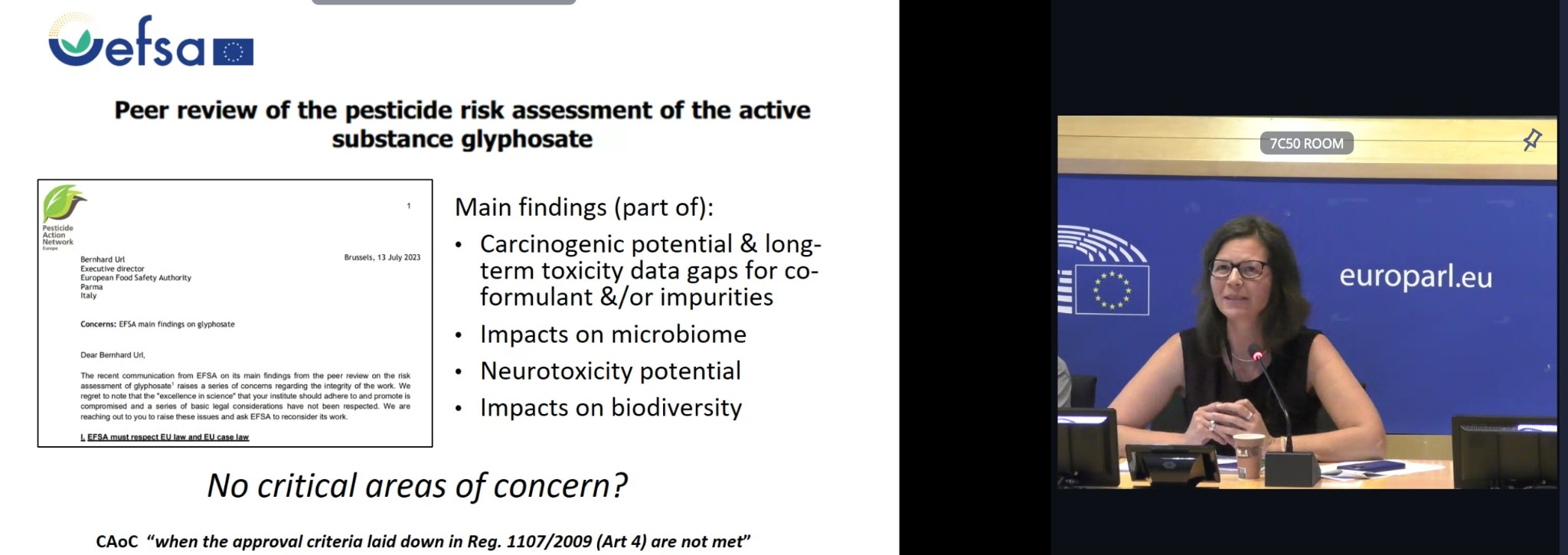
Dr Lysimachou highlighted that the presentations by the previous speakers already explained that glyphosate active substance should be considered a presumed carcinogen, based on the correct analysis of animal studies, epidemiology studies and mechanistic evidence (e.g. oxidative stress). In addition EFSA identified data gaps and pending issues during its peer review of glyphosate risk assessment that should have been identified as Critical Areas of Concern according to EU and case law. A focus was given to the following: the potential genotoxicity of an impurity present in the products, the missing toxicity data of one co-formulant, the evidence on neurotoxicity, microbiome alterations and impacts on biodiversity following exposure to glyphosate-based herbicides. This was already communicated to EFSA in a letter.
In EFSA’s peer review and also in the reply to our letter, EFSA explains “there was no indication that the formulation MON 52276 would exhibit long term toxicity. Nevertheless the long term toxicity of this formulation has not been tested by the applicants. In fact the evidence from independent research point in the opposite direction from EFSA’s position as explained by previous speakers: they indicate that in laboratory animals MON 52276 may alter the microbiome, cause genotoxicity and hepatic toxicity, and has been found to cause reproductive organ abnormalities indicating endocrine disruption.
If we look at the dossier in the mammalian toxicology section, and check the number of studies submitted by the applicants on the active substance versus the ones on the formulation MON 52276 we see that there are zero long-term toxicity studies done on the formulation. However, at least two impurities of the formulation have been found to be toxic (potentially genotoxic or carcinogenic), and long term toxicity studies for all the ingredients are missing. With the indications from the scientific literature indicating serious adverse effects following exposure to glyphosate-based herbicides it is a violation of the EU law to renew the approval of glyphosate. Furthermore, the EU case law (Blaise ruling) explains that in case of uncertainty the Regulators can proceed with taking measures.
Therefore, considering all the evidence from the peer-reviewed scientific literature, and the evidence provided on the assessment of the cancer studies in this meeting, our EU regulation should at the very minimum consider there is a level of uncertainty on the safety of glyphosate. According to EU law and EU case law, glyphosate’s approval should not be renewed as a high level of protection from human health and the environment has not been demonstrated.
Presentation: A civil society perspective
See the RECORDING in French & RECORDING in English