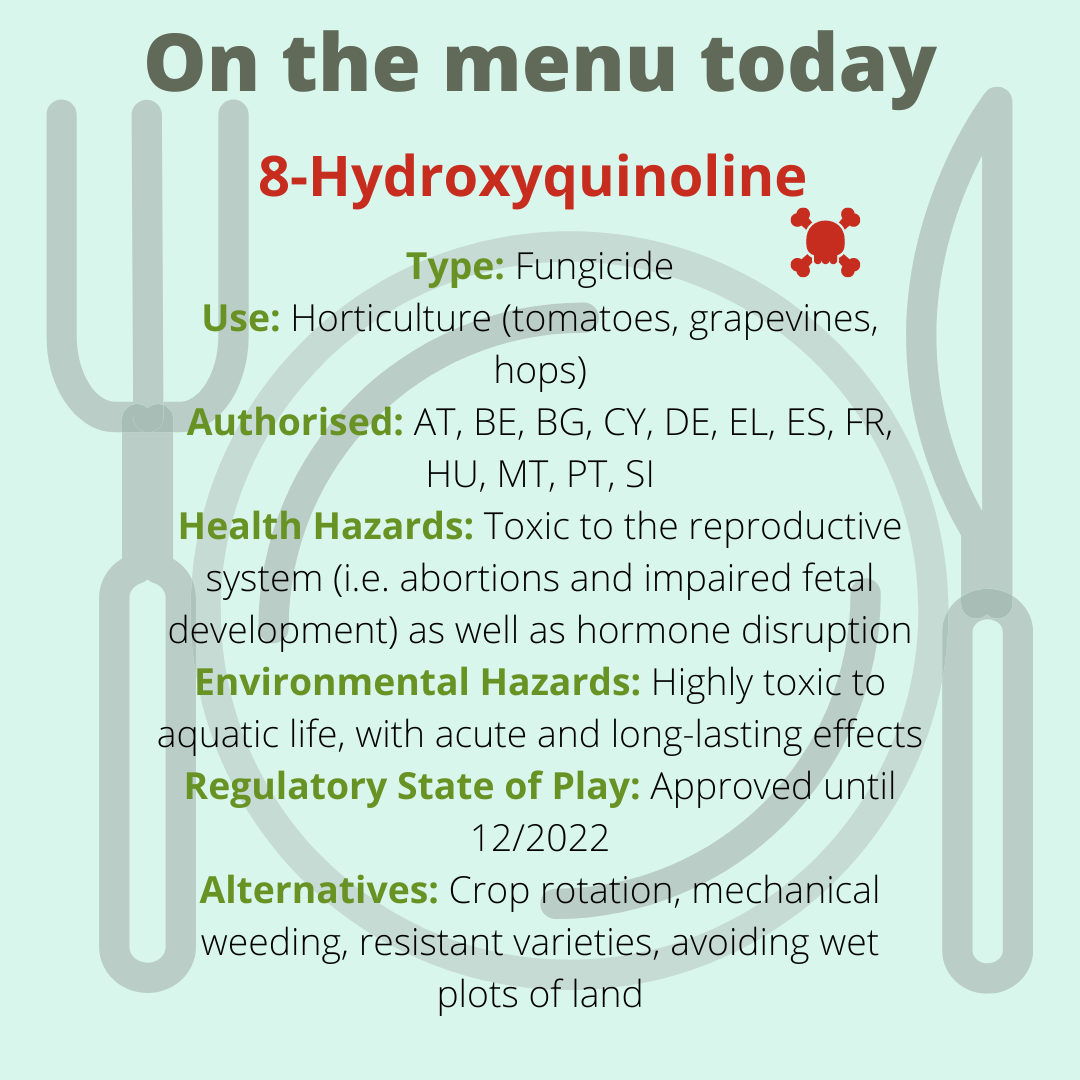
The European Commission is using a dangerous concept to renew the authorisation of a very harmful pesticide that can damage the unborn child. For the very first time, it plans to use an exceptional clause of the Regulation called ‘negligible exposure’. It basically says: “Yes, it’s very toxic, but don’t worry, nobody will be exposed to it.” However, there is no reliable and realistic scientific evidence for this claim that would allow the continued use of the ‘Toxic 12’ fungicide 8-hydroxyquinoline.
The Commission proposes the renewal of the ‘reprotoxic’ fungicide 8-hydroxyquinoline, based on the claim of "negligible exposure” to humans. This exceptional clause of negligible exposure allows for the approval of highly hazardous pesticides if it can be convincingly proven that there will be no contact with humans according to the Pesticide Regulation. Worryingly, such a robust demonstration was not provided for 8-hydroxyquinoline. PAN Europe is calling upon Member States to reject the Commission’s proposal and ban 8-hydroxyquinoline to protect human health.
Classified as toxic for reproduction category 1B since 2015, 8-hydroxyquinoline is presumed to "damage the unborn child." Under the EU Pesticide Regulation, substances that present such hazards cannot be approved. An exception is possible if negligible exposure to humans can be convincingly demonstrated. Negligible exposure should be strictly understood as conditions of use excluding contact with humans and resulting in no residues of the pesticide in food.
Member States and the Commission have not reached a consensus yet on a guidance document to assess and define negligible exposure in the course of pesticide risk assessment. As a result, this clause of negligible exposure has never resulted in the approval of a hazardous pesticide since the Pesticide Regulation came into force in 2011.
This situation could change as the Commission is now proposing to renew 8-hydroxyquinoline in permanent greenhouses by drip-irrigation building on the negligible exposure clause. Worryingly, the data presented by the applicant and summarised in EFSA's peer review conclusions fall short of the rigorous scientific standards required to justify the renewal of this hazardous substance and do not present realistic use conditions.
For substances known or presumed to be genotoxic or endocrine disruptors, no threshold is considered safe, particularly for pregnant women, newborns and children. For this precise reason, the use of the ‘negligible exposure’ could completely twist the purpose of the Regulation, which is to remove harmful substances from the EU market rather than to try to mitigate the related risks.
Unreliable study and unrealistic scenario as cornerstones for far-reaching proposal
Negligible exposure to 8-hydroxyquinoline must be demonstrated for four exposure groups: operators, workers, residents and bystanders. For this, the applicant submitted anon-dietary exposure study for workers and operators. According to EFSA, this was plagued by technical deficiencies, rendering it unreliable for quantitative risk assessment. Despite this, the study formed the cornerstone of the argument that exposure levels are acceptable for operators and workers in case of automated drip-irrigation only if operators wear protective equipment and are not exposed during the application phase. If operators find themselves exposed during application, for example due to a technical incident, the negligible exposure level is largely exceeded. For bystanders’ and residents’ children, EFSA concluded that the level of negligible exposure was exceeded for spray application and was unable to refine its assessment for drip irrigation’s use due to lack of robust data in the study.
In addition to these major flaws that do not allow robust scientific conclusions to be drawn, this study does not take into account realistic conditions of use. As a result, it completely underestimates actual exposure to this substance. For example, the study assumes that the greenhouse inside and outdoor temperatures are approximately 20°C. In fact, the greenhouse temperature in southern European countries is about 40°C. Since the volume of vapour pressure of 8-hydroxyquinoline is dependent on the temperature, the level of negligible exposure is highly exceeded according to the calculation of one Member State. Likewise, the study is based on a scenario involving the application of a maximum volume of 8-hydroxyquinoline of 600 grams over an area of 0.5 hectares. Many greenhouses are much larger and therefore require the mixing, loading and application of larger quantities of 8-hydroxyquinoline.
Discussion with EU Member States on 2 and 3 October
The Commission’s proposal will be discussed with Member States on 2 and 3 October. More discussions and possibly a vote will take place in late 2024 or the beginning of 2025. Meanwhile, the current approval period of the substance will be extended. Since 2021, 8-hydroxyquinoline is on PAN Europe's list of Toxic12 pesticides to be banned immediately.
PAN Europe urges Member States to reject the renewal of 8-hydroxyquinoline and to advocate for the immediate withdrawal of products containing this substance from the EU market. This is not just a regulatory matter; it is a critical public health issue that demands decisive action.
Read more: